Do some people with type 1 diabetes have a rougher ride than others?
Ever heard of
“Brittle diabetes”
I used to think, stop making excuses.
Why?
I consistently have 99% time in range (3.9mmol/L – 10.0mmol/L) using Dynamic Glucose Management.
So obviously, anyone not achieving at least 70% time in range must not be trying too hard, right?
Wrong!
Over the last ten years, I have supported numerous young people and their families who cannot reach 70% time in range despite using Dynamic Glucose Management, carb counting every meal, dialling in advanced bolusing and monitoring their CGM like a hawk.
Seeing their almighty struggle smashed my “self-righteous small-minded” thinking, and forced me to ask the question:
Are there different types of type 1 diabetes?
This question has consumed me for the last couple of years. Here is my current thinking after reviewing the research.
In my view, it revolves around how much residual insulin production capacity the b-cells of the pancreas retain immediately after diagnosis and much longer after diagnosis.
People with type 1 diabetes who retain more residual pancreatic b-cell function in the first few years after diagnosis have lower HbA1cs, fewer glucose ups and downs, reduced risk of DKA, and less frequent severe hypoglycaemia (1).
Once the honeymoon subsides, usually defined as two years after diagnosis (2), a higher residual b-cell function protects against severe hypoglycaemia (3), microvascular complications, DKA (4), glucose excursions after meals and overnight (5) and time out of range after exercise (6).
This begs the question.
How do you find out how much residual function is left in the b-cells?
Let me introduce C-peptide.
Before insulin is released from the b-cell, it must be cleaved from C-peptide. For every one molecule of insulin, there is one molecule of C-peptide.
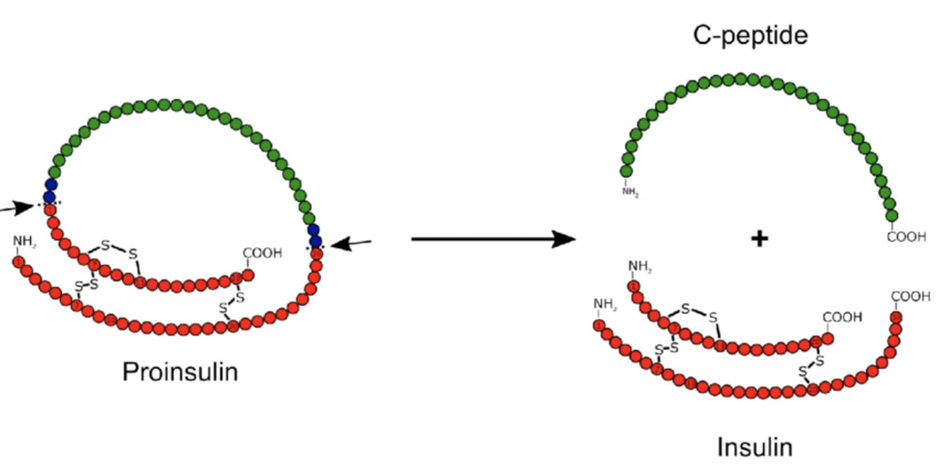
Once separated, insulin and C-Peptide are released into the portal vein. Half of the insulin is used immediately in the liver to store glucose. However, all the C-peptide escapes the liver into the external blood supply, eventually being broken down or excreted by the kidneys.
This means C-peptide is a better measure of how much insulin the b-cells are producing at the time of testing. Insulin injected by a pen or infused by a pump does not have a C-peptide attached.
Therefore, C-peptide is an effective measure of how much residual insulin production people with type 1 diabetes have.
Simply, C-peptide tells a person with type 1 diabetes how much help they do or do not get from their pancreas.
I bet you are wondering.
What level of C-peptide is needed to have?
- lower HbA1c
- less diabetes-related long-term complications
- less DKA
- less severe hypos
- Lower total daily insulin dose
- More time in range
- Fewer issues managing glucose control after exercise
Let’s not get ahead of ourselves.
We must first discuss how to test for C-peptide.
How to test for C-peptide.
There are a few C-peptide tests that can be used (7):
- Glucagon Stimulated Test (GST). The person gets an injection of 1mg Glucagon. C-Peptide is measured over two, four, and six minutes after the Glucagon injection. The peak measurement is taken.
- Mixed Meal Tolerance Test (MMTT): Consume a liquid meal (carbs, fat, and protein) based on weight with no bolus insulin and measure C-peptide over the next two hours. The peak measurement is taken.
- Urinary C-peptide to creatine ratio (UCPCR): One-off urine sample. The peak measurement is taken.
- Fasting C-peptide (FC): C-peptide measured fasting.
- Random C-peptide (RC): Random blood test and measured C-peptide once.
Wow, which one to go for?
The GST and MMTT are the tests used most frequently in the research. However, outside a research trial, it is unlikely to get one.
UCPCR has shown to be accurate (8) and only requires one urine sample, although it’s not as popular as blood test methods in the research.
That leaves fasting C-peptide or random C-peptide, which one to request?
Research shows that random C-peptide is the most accurate when compared to MMTT, with a very tight correlation of 0.91 for my nerdy friends (9). Fortunately, this is the easiest one to do as it requires no preparation and is widely available.
Just make sure you have eaten a decent mixed meal in the two hours before and only have given half of the usual insulin, at most. This will ensure your glucose rises and the b-cells will be stimulated to pump out insulin if you have a residual function.
What are the cut-offs used to define a residual b-cell function?
Absolute cut-offs for C-peptide differ slightly between studies but can be categorised broadly as (3).
- HIGH residual function (>0.2nmol/L or >200pmol/L)
- INTERMEDIATE residual function (0.03 – 0.2nmol/L or 30 to 200pmol/L))
- LOW residual function (<0.03nmol/L or <30pmol/L)
To put this into perspective, people without type 1 diabetes have a level of 1-3nmol/L or 1000 – 3000pmol/L after eating (10).
Even if you are categorised as HIGH, it’s only about 10% of fully functioning b-cells.
Does it matter how long after diagnosis C-peptide is tested, and the age of diagnosis?
You bet it does.
Data from 610 type 1’s aged 13-39 from the DCCT was investigated to answer this question. It was found 1-5 years after diagnosis that up to 48% still have a HIGH function. However, by 5-15 years after diagnosis, only 8% retain HIGH function (11).
Unfortunately for the people diagnosed aged 13-18 years, none were categorised as HIGH after 5-15 years (11).
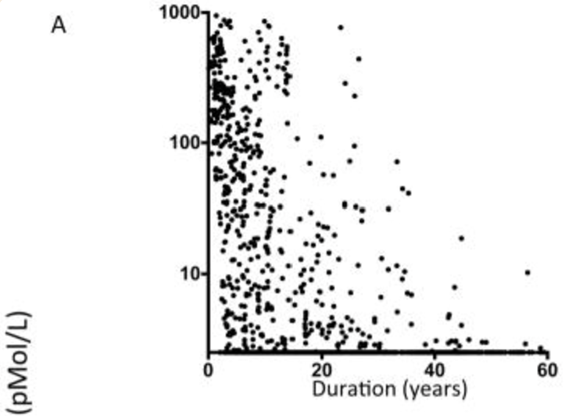
These results have been replicated in the Massachusetts General Hospital. They looked at 1273 patients with Type 1 diabetes aged 8-90 years (12). Each participant had a fasting C-peptide measured at one point during their diabetes journey. The investigators then assessed if fasting C-peptide declines the longer you live with diabetes.
Graph A below shows that C-Peptide declines progressively the longer a person has diabetes.
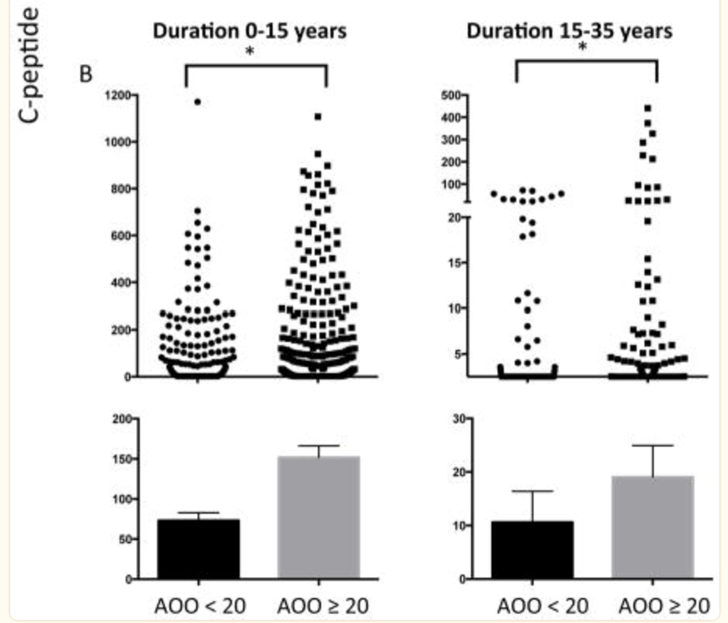
They also want to know if fasting C-peptide is lower when the diagnosis age is under the age of 20 years (AOO<20). Specifically, if diabetes duration has been 0-15 years or 15-35 years does age of diagnosis impact fasting C-peptide.
Graph B shows significantly lower C-Peptide for people diagnosed under the age of 20 at both diabetes durations (12).
Coming back to reality.
If you have had type 1 diabetes for at least five years you can be reasonably confident of your categorisation of HIGH, INTERMEDIATE or LOW.
However, if you are only two years into diagnosis, your residual b-cell function will likely drop quite a bit further, and another test later may be warranted. If you were diagnosed under the age of 20, it’s more likely you will be in the INTERMEDIATE of the LOW group, but it’s not certain.
Time to fess up!
I had my C-peptide tested recently, more than 13 years post-diagnosis by random C-peptide.
I ate a mixed meal with only half my usual insulin and got a random C-peptide test.
What was the result?
0.22nmol/L or 220pmol/L – I am in the HIGH category.
This is not surprising considering I was diagnosed aged 27 and only take 30 units of insulin a day despite being 100kg.
I used to think that only requiring 0.3 units of insulin per kilogram was due solely to my high level of activity. In reality, my residual b-cell function is at play here too.
Ok, a quick recap:
- C-peptide tells us how much help we get from the remaining b-cells
- It can be tested two hours after a mixed meal by a random blood test
- The result can be categorised as HIGH, INTERMEDIATE, or LOW
- If tested within five years of diagnosis it will likely drop further over time
- If you were diagnosed under the age of 20, you will very likely have an INTERMEDIATE or LOW function.
Let’s dig into the studies about health outcomes.
Published in 1987, the landmark DCCT trial looked at 610 patients who had C-peptide results. They found those with HIGH function had lower HbA1cs, lower fasting glucose levels, and lower total daily insulin dose compared to those with LOW function at seven years of follow-up (11).
A more recent DCCT report confirmed the benefit persists. The HIGH group had lower HbA1cs, lower insulin doses, and a lower risk of eye disease 35 years later compared to the LOW group (13). This report showed a linear relationship between C-peptide and improved clinical outcomes.
Most recently, the HIGH and INTERMEDIATE residual b-cell function groups had significantly fewer episodes of severe hypoglycaemia compared to the LOW group (3).
This all simply means, that the higher the C-Peptide, the lower the risk of future complications related to diabetes (13).
What about day-to-day control – time in range (3.9-10.0mmol/L or 70-180mg/dL)?
A team in Newcastle (England) followed 66 people with type 1 in free-living conditions after categorisation by C-peptide (5). After controlling for the important variables, they found the HIGH group had 76% time in range overnight compared to 58% for the LOW group. The high group had 68% time in range after meals compared to 51% for the LOW group (5).
The same group looked at the impact of residual b-cell function on after exercise time in range. They categorised 30 adults with type 1 diabetes by residual b-cell function and had them complete 45 minutes of incline treadmill walking at a pace causing heavy breathing (6). In the 12 hours after exercise, the HIGH group had 73% time in range compared to 44% and 41% for the INTERMEDIATE and LOW groups respectively (6).
Also, the HIGH group had lower glucose variability, less time above 13.9mmol/L (250mg/dL) and a trend toward less time below 3.9mmol/L (70mg/dL) compared to the INTERMEDIATE and LOW groups.
Finally, the change in time in range from pre-exercise to 24-h after exercise was as expected. The groups with LOW and INTERMEDIATE function experienced worsening time in range, whereas the HIGH group improved time in range by 12.1% (6).
To summarise, the more residual function you have, the more in target the glucose will be overnight, after eating and after exercise.
How does having a higher C-peptide help?
There are lots of theories.
Firstly, Insulin from the b-cell is delivered straight into the portal vein allowing a lot of the glucose from the meal to be squirrelled away into the liver as glycogen. This reduces after-meal highs. This is a direct effect of residual insulin on the glucose level.
Secondly, it has been suggested that residual b-cell function stops the release of glucagon when the glucose rises, which unhelpfully happens in people with type 1 diabetes but not for people without (14). Remember Glucagon causes the liver to release gluocse that is stored as glycogen.
This can cause the glucose to spiral out of control with higher glucose then leading to more Glucagon released.
Specifically, it has been theorised the insulin from the b-cell puts the break on the a-cell putting out glucagon in response to rising glucose levels (14). Higher levels of glucagon have been reported in groups with HIGH versus low function adding some support to this.
The benefits of residual b-cell function are likely to be multifactorial and more research is required to elucidate the mechanisms.
Simply, residual b-cell function helps, we are not clear exactly why just yet.
Recently a study using exercise as the stimulus looked at the difference between cardio-protective haematopoietic and endothelial progenitor cells between HIGH and LOW groups (16). Results showed the HIGH group increased protective cells in response to exercise to the same magnitude as non-diabetic controls. Whereas the LOW group did not experience any rise from rest (16).
This suggests that residual b-cell function boosts the benefits of exercise too!
Why does knowing your category help?
If the residual b-cell function is LOW or INTERMEDIATE, there is no need to panic and feel helpless. It simply requires some creativity.
For example.
Knowing if a person with type 1 diabetes has HIGH, INTERMEDIATE or LOW residual function is a valuable tool to triage advanced therapies such as:
- Artificial insulin delivery systems
- GLP-1 therapies (17)
- Amylin
- Bespoke nutritional strategies
- Individualized exercise strategies
- Dynamic Glucose Management
- Mealtime Insulin Guide
I recently had success supporting a young triathlete with LOW function using bespoke nutritional strategies such as:
- Low carb meal prior to exercise to prevent starting exercise with high glucose and minimise circulating insulin.
- Doing most of the carb-loading after exercise when insulin sensitivity is high.
- Small amounts of glucose every 10-15 minutes during exercise based on glucose value and trend arrow, rather than larger amounts every 30 minutes.
- Bespoke before bed management algorithm aimed at tight control before bed.
This is outside the box thinking that has taken time in range from 30% to 60% and improved glucose levels during exercise and boosted performance. Next, we are considering a T-Slimx2 with Control IQ overnight and back to injections during the day.
We also know that b-cell function declines over time. Is there anything that can be done to slow this process?
Of course,
Maintaining as much time in range as possible reduces oxidative damage of the b-cells from high glucose levels. Using Individualized exercise strategies, Dynamic Glucose Management, the Mealtime Insulin Guide and anything that keeps the glucose in range will help.
Emerging research suggests that being as active as possible reduces inflammation and may preserve b-cell function.
Lastly, emerging immunotherapies are showing some promise in preserving b-cell function in people newly diagnosed with type 1 diabetes.
Empathy?
C-peptide is a tool of empathy. Knowing the C-peptide level category should help diabetes teams, people with type 1 and caregivers understand why diabetes management can be such a challenge for some despite trying extremely hard.
This will be especially useful for people in the LOW group who compare themselves to someone like me in the HIGH group. It’s like comparing chalk with cheese and unfair to the person in the LOW group.
I hope this has helped you in some way.
This deep dive smashed my old perspective.
I used to think everyone with Type 1 diabetes could get 70% or more time in range if they just tried hard enough!
I no longer believe this.
It has given me greater empathy for people with type 1 diabetes and their struggles.
But most importantly, it has informed me there is a test we can use to categorise and triage therapies more effectively.
I wonder if this will become the standard of care one day?
Here’s hoping.
References
1. Abdul-Rasoul M, Habib H, Al-Khouly M. “The honeymoon phase” in children with type 1 diabetes mellitus: frequency, duration, and influential factors. Pediatr Diabetes. 2006 May;7(2):101–7.
2. Schölin A, Berne C, Schvarcz E, Karlsson FA, Björk E. Factors predicting clinical remission in adult patients with type 1 diabetes. J Intern Med. 1999;245(2):155–62.
3. Gubitosi-Klug RA, Braffett BH, Hitt S, Arends V, Uschner D, Jones K, et al. Residual β cell function in long-term type 1 diabetes associates with reduced incidence of hypoglycemia. J Clin Invest. 2021 Feb 1;131(3).
4. Jeyam A, Colhoun H, McGurnaghan S, Blackbourn L, McDonald TJ, Palmer CNA, et al. Clinical Impact of Residual C-Peptide Secretion in Type 1 Diabetes on Glycemia and Microvascular Complications. Diabetes Care. 2021 Feb 1;44(2):390–8.
5. Taylor GS, Shaw AC, Smith K, Wason J, McDonald TJ, Oram RA, et al. Capturing the real-world benefit of residual β-cell function during clinically important time-periods in established Type 1 diabetes. Diabet Med. 2022 May 1;39(5).
6. Taylor GS, Smith K, Capper TE, Scragg JH, Bashir A, Flatt A, et al. Postexercise Glycemic Control in Type 1 Diabetes Is Associated With Residual β-Cell Function. Diabetes Care. 2020 Oct 1;43(10):2362–70.
7. Leighton E, Sainsbury CA, Jones GC. A Practical Review of C-Peptide Testing in Diabetes. Diabetes Ther. 2017 Jun 1;8(3):475–87.
8. McDonald TJ, Knight BA, Shields BM, Bowman P, Salzmann MB, Hattersley AT. Stability and reproducibility of a single-sample urinary C-peptide/creatinine ratio and its correlation with 24-h urinary C-peptide. Clin Chem. 2009 Nov 1;55(11):2035–9.
9. Hope S V., Knight BA, Shields BM, Hattersley AT, McDonald TJ, Jones AG. Random non-fasting C-peptide: bringing robust assessment of endogenous insulin secretion to the clinic. Diabet Med. 2016;33(11):1554–8.
10. Yosten GLC, Maric-Bilkan C, Luppi P, Wahren J. Physiological effects and therapeutic potential of proinsulin C-peptide. Am J Physiol Endocrinol Metab. 2014 Dec 1;307(11):955–68.
11. Group TDR. Effects of age, duration and treatment of insulin-dependent diabetes mellitus on residual beta-cell function: observations during eligibility testing for the Diabetes Control and Complications Trial (DCCT). The DCCT Research Group. J Clin Endocrinol Metab. 1987;65(1):30–6.
12. Kuhtreiber WM, Washer SLL, Hsu E, Zhao M, Reinhold P, Burger D, et al. Low levels of C-peptide have clinical significance for established Type 1 diabetes. Diabet Med. 2015 Oct 1;32(10):1346.
13. Lachin JM, McGee P, Palmer JP. Impact of C-peptide preservation on metabolic and clinical outcomes in the Diabetes Control and Complications Trial. Diabetes. 2014 Feb;63(2):739–48.
14. Guo K, Tian Q, Yang L, Zhou Z. The Role of Glucagon in Glycemic Variability in Type 1 Diabetes: A Narrative Review. Diabetes Metab Syndr Obes. 2021 Dec;14:4865–73.
15. Thivolet C, Marchand L, Chikh K. Inappropriate glucagon and GLP-1 secretion in individuals with long-standing type 1 diabetes: effects of residual C-peptide. Diabetologia. 2019 Apr 1;62(4):593–7.
16. Taylor GS, Shaw A, Scragg JH, Smith K, Campbell MD, McDonald TJ, et al. Type 1 Diabetes Patients With Different Residual Beta-Cell Function but Similar Age, HBA1c, and Cardiorespiratory Fitness Have Differing Exercise-Induced Angiogenic Cell Mobilisation. Front Endocrinol (Lausanne). 2022 Feb 11;13.
17. Mathieu C, Zinman B, Hemmingsson JU, Woo V, Colman P, Christiansen E, et al. Efficacy and Safety of Liraglutide Added to Insulin Treatment in Type 1 Diabetes: The ADJUNCT ONE Treat-To-Target Randomized Trial. Diabetes Care. 2016 Oct 1;39(10):1702–10.
